Deep-diving whales could help trauma patients. Here's how
Digital Reporter
Sunday, September 27, 2015, 9:39 AM - Oxygen-binding proteins found in the muscle tissue of deep-diving whales could hold the lifesaving answer for human trauma patients, according to a new study.
While the protein myoglobin is found in humans, whales and other mammals, the study found that marine creatures have ultra-stable versions of the protein.
The study published in the Journal of Biological Chemistry by Rice University biochemists, found that stability was the key for cells to make large quantities of myoglobin, which explains why whales hold far more of the protein in their muscle cells than humans. This in turn, allows whales to hold their breath for up to two hours.
"Whales and other deep-diving marine mammals can pack 10 to 20 times more myoglobin into their cells than humans can," biochemist John Olson said in a Rice University press release. "The reason why whale meat is so dark is that it's filled with myoglobin that is capable of holding oxygen. But when the myoglobin is newly made, it does not yet contain heme. We found that the stability of heme-free myoglobin is the key factor that allows cells to produce high amounts of myoglobin."
![]() RELATED: Curious whale interrupts research team's live video feed
RELATED: Curious whale interrupts research team's live video feed
Olson has spent about 20 years studying the protein, and wants to create a strain of bacteria that can create massive amounts of another protein that is similar to myglobin. The goal is to develop a synthetic blood for use in transfusions.
Donated whole blood is often in short supply and has a short shelf life and Olson's aim is to maximize the amount of hemoglobin that a bacterium can express, the release reads. A vitro method was developed by researches to test myoglobin expression outside of living cells.
"That allowed us to carefully control all the variables," said Olson. "We found that the amount of fully active myoglobin expressed was directly and strongly dependent on the stability of the protein before it bound the heme group."
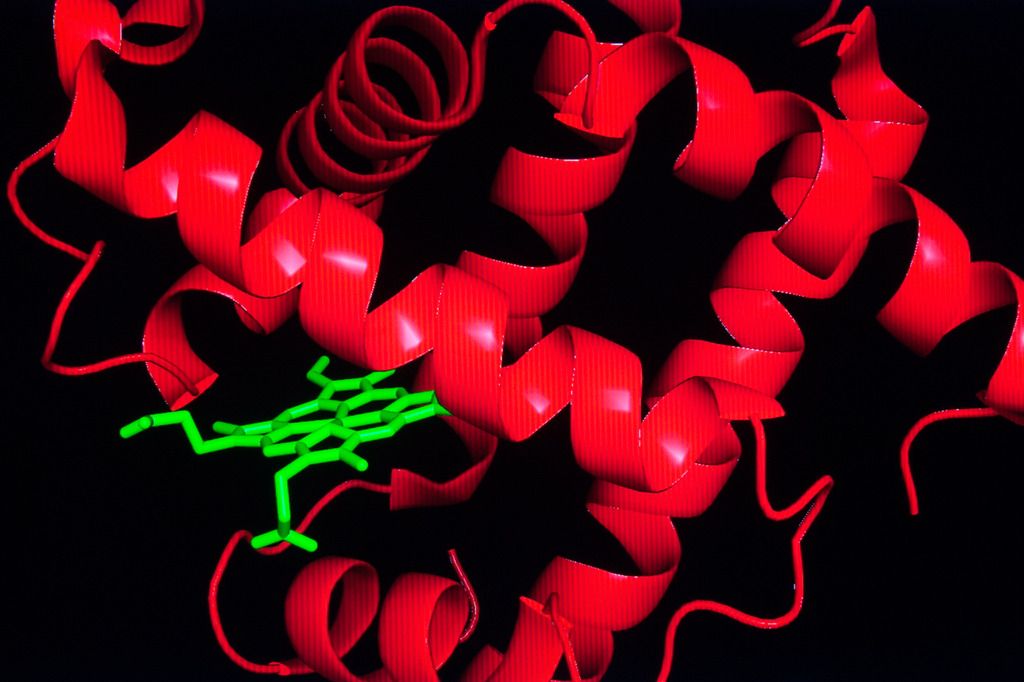
Researchers studied the protein's stability and resistance to unfolding by analyzing the overall structures and shape of myoglobin from different species.
Stability in myoglobin was studied in humans, pigs, goosebeak whales, gray seals and sperm whales to name a few of the species.
Stability was measured by using chemicals that forced proteins to unfold. A graduate student in Olson's lab observed how myoglobin in sperm whales was much more resistant to chemically induced unfolding, unlike human or pig proteins. It was confirmed in 2000 that resistance to unfolding was a trait of deep-diving whales.
Source: Rice University | Study
Related video: Amazing encounter with humpback whale



